
 Semin Immunol. 2023研究手段:免疫肽组学早期的时候,科学家试图通过MHC/HLA识别的多肽序列特征,预测免疫肽组的组成,但是符合每个MHC/HLA共识序列基序的可能肽序列比实际提呈的要多得多,因此,预测免疫肽组实际组成的尝试大多失败了。现在,研究手段与蛋白质组类似,以质谱分析为主。MHC分子的免疫亲和纯化开始,然后提取结合肽,首先通过Edman降解,再通过串联质谱进行分析。
Semin Immunol. 2023研究手段:免疫肽组学早期的时候,科学家试图通过MHC/HLA识别的多肽序列特征,预测免疫肽组的组成,但是符合每个MHC/HLA共识序列基序的可能肽序列比实际提呈的要多得多,因此,预测免疫肽组实际组成的尝试大多失败了。现在,研究手段与蛋白质组类似,以质谱分析为主。MHC分子的免疫亲和纯化开始,然后提取结合肽,首先通过Edman降解,再通过串联质谱进行分析。
Semin Immunol. 2023
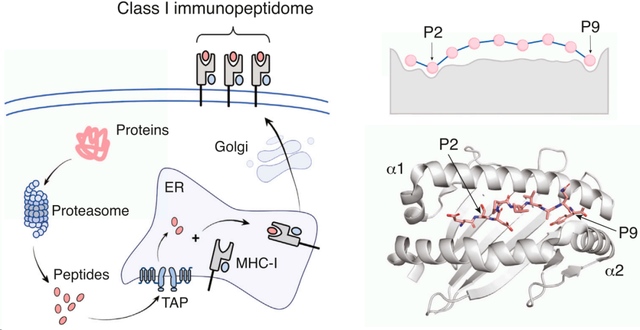
一旦进入内质网,多肽就可以加载到 MHC-I 分子上,这些分子与β2m形成异二聚体,并且只有在与肽结合时才能稳定地折叠成复合物,最后将稳定的肽-MHC-I复合物易位到细胞膜上,MHC-I肽结合位点位于细胞外环境中。与MHC-I不同,MHC-II分子主要在抗原呈递细胞中表达,如B细胞、巨噬细胞或树突状细胞。MHC-II分子上展示的多肽可以来自外源性和内源性蛋白质。这些肽通过内吞或吞噬途径加工。MHC-II 分子形成二聚体,具有 α 链和 β 链。MHC-II二聚体通常首先结合不变链,然后进一步裂解成CLIP肽。当与晚期内体中的其他肽相遇时,CLIP肽被其中一些肽取代,肽-MHC-II复合物被转移到细胞表面。该过程由多个伴侣控制,包括人类的HLA-DM和HLA-DO。
Semin Immunol. 2023
MHC-II分子的结合位点与MHC-I分子的结合位点相似,但两端更开放。因此,MHC-II配体的特征在于结合核心,其侧翼残基延伸在结合核心的两侧,一般为12-25个残基的多肽。自身免疫多肽组
同源MHC-多肽配体的 T 细胞识别,不是一个on/off的二元开关,因为 TCR 可以“感知”最佳和次优配体之间的差异 。强激动剂多肽,即使在低纳摩尔浓度下,也可以刺激CD4+和CD8+ T细胞的增殖和效应功能(细胞因子产生、细胞毒性反应)。部分激动剂,则需要更高的浓度才能诱导相同的 T 细胞反应和效应淋巴因子的分泌,拮抗剂多肽,通过 TCR 接触点的单个氨基酸取代,特异性抑制激动剂诱导的反应。因此,CD4+ 和CD8+ T 细胞上存在的 α/β TCR可以区分MHC 肽构象中的细微结构变化,并将同源配体识别的affinity/avidity转化为不同的 T 细胞反应。TCR 在肽-MHC 结合后,产生不同信号,指导 Tconv 和 Treg 发育。举例:在2 型糖尿病 (T2D) 中, PDIA3 和 ApoB 肽均可被 Tconv 和Treg 识别。在生理条件下,MHCII类分子I-Ab 呈递了大约 0.4 飞摩尔的PDIA3 肽和 0.05 飞摩尔的 ApoB 肽,但在代谢异常条件下,由于高脂肪、高蔗糖饮食,PDIA3 和 ApoB 表位的MHCII类分子I-Ab 呈递增加 40% 。肿瘤免疫多肽组
肿瘤免疫多肽组,是个性化肿瘤多肽疫苗/mRNA疫苗设计的重要基础。举例:NCT02149225:对新诊断的胶质母细胞瘤进行积极个性化疫苗接种,相关数据发表于Nature。
Nature 2019
他们建立了一个免疫肽组学引导的“现成”仓库,包括多种胶质母细胞瘤相关肿瘤抗原。他们根据每个患者个体肿瘤的免疫肽组和转录组,以及疫苗前T细胞对潜在靶标的反应性,为每位患者选择了相关的仓库肽。通过这种方式,对仓库肽进行患者特异性排名,以选择七种最合适、排名最高的 HLA I 类肽。其中,添加了两个 HLA-DR 肽和一个病毒对照标记肽,构成主动个性化疫苗 1 (APVAC1)。部分在招募中临床研究NCT #Study TitleSponsorCollaboratorsNCT05916261Personalized Tumor Vaccines and Pabolizumab in Patients With Advanced Pancreatic CancerRuijin HospitalNCT05916248Personalized Tumor Vaccines and Pembrolizumab in Patients With Advanced Solid TumorsRuijin HospitalShanghai Xinpu BioTechnology Company LimitedNCT05359354Safety and Efficacy of Personalized Neoantigen Vaccine in Advanced Solid TumorsYueJuan ChengNeoCuraNCT05475106Pilot Study of Neoantigen Peptides and Leukine for the Treatment of NeoplasmsInstituto de Medicina RegenerativaNCT03908671Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen in Patients With Advanced Esophageal Cancer and Non-small Cell Lung CancerStemirna TherapeuticsThe First Affiliated Hospital of Zhengzhou UniversityNCT05749627Using Neoantigen Peptide Vaccine/Neoantigen-based DC to Treat Advanced Malignant Solid TumorsThe First Affiliated Hospital of Nanchang UniversityShanghai Dengding BioAI Co.NCT03558945Clinical Trial on Personalized Neoantigen Vaccine for Pancreatic TumorAnda Biopharmaceutical Development (Shenzhen) Co., Ltd.NCT05111353Neoantigen Vaccines in Pancreatic Cancer in the Window Prior to SurgeryWashington University School of MedicineNational Cancer Institute (NCI)|Leidos|UNICO FoundationNCT05192460Safety and Efficacy of Personalized Neoantigen Vaccine in Advanced Gastric Cancer, Esophageal Cancer and Liver Cancerjianming xuNeoCuraNCT04749641Neoantigen Vaccine Therapy Against H3.3-K27M Diffuse Intrinsic Pontine GliomaYang ZhangTCRCure Biopharma Ltd.NCT03219450A Personalized Neoantigen Cancer Vaccine in Treatment Na茂ve, Asymptomatic Patients With IGHV Unmutated CLL.Dana-Farber Cancer InstituteOncovir, Inc.|BioNTech SE|Merck Sharp & Dohme LLCNCT02950766NeoVax Plus Ipilimumab in Renal Cell CarcinomaPatrick Ott, MD, PhDBristol-Myers Squibb|Oncovir, Inc.NCT05767684Neoantigen Derived DCs as Cancer TreatmentNational Health Research Institutes, TaiwanNational Cheng-Kung University HospitalNCT05307835Neoantigen Vaccine in Esophagus Cancer Patients Following Neoadjuvant Therapy and Surgical ResectionSecond Affiliated Hospital, School of Medicine, Zhejiang UniversityHangzhou Neoantigen Therapeutics Co., Ltd.NCT03361852Personalized Neoantigen Cancer Vaccine + Pembrolizumab After Rituximab for Follicular LymphomaDana-Farber Cancer InstituteNCT06019702Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen Alone in Subjects With Advanced Digestive System NeoplasmsSir Run Run Shaw HospitalHangzhou Neoantigen Therapeutics Co., Ltd.NCT05198752A Study of Neoantigen mRNA Personalised Cancer in Patients With Advanced Solid TumorsStemirna TherapeuticsNCT05940181A Safety and Efficacy Study of XH001 Combined With Sintilimab Injection in Advanced Solid Tumorsjianming xuNeoCuraNCT02287428Personalized NeoAntigen Cancer Vaccine w RT Plus Pembrolizumab for Patients With Newly Diagnosed GBMDana-Farber Cancer InstituteThe Ben & Catherine Ivy Foundation|Accelerate Brain Cancer Cure|Merck Sharp & Dohme LLC|National Institutes of Health (NIH)NCT04810910Personalized Neoantigen Vaccine in Pancreatic Cancer Patients Following Surgical Resection and Adjuvant ChemotherapyZhejiang Provincial People's HospitalHangzhou Neoantigen Therapeutics Co., Ltd.NCT06026774Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen in Subjects With Resected Digestive System NeoplasmsSir Run Run Shaw HospitalHangzhou Neoantigen Therapeutics Co., Ltd.NCT05444530A Study of VAC85135, a Neoantigen Vaccine Regimen, Concurrently Administered With Ipilimumab for the Treatment of Myeloproliferative NeoplasmsJanssen Research & Development, LLCBristol-Myers SquibbNCT04912765Neoantigen Dendritic Cell Vaccine and Nivolumab in HCC and Liver Metastases From CRCNational Cancer Centre, SingaporeBristol-Myers SquibbNCT04864379Clinical Study of a Personalized Neoantigen Cancer Vaccine Combined With Anti-PD-1 and RFA in Patients With Solid TumorsSir Run Run Shaw HospitalHangzhou Neoantigen Therapeutics Co., Ltd.NCT04968366Safety & Efficacy of DC Vaccine and TMZ for the Treatment of Newly-diagnosed Glioblastoma After SurgeryBeijing Tiantan HospitalZhongSheng BioTech Inc.NCT05078866Cancer Preventive Vaccine Nous-209 for Lynch Syndrome PatientsNational Cancer Institute (NCI)NCT06026800Clinical Study of Personalized mRNA Vaccine Encoding Neoantigen in Combination With Standard First-line Treatment in Subjects With Advanced Digestive System NeoplasmsSir Run Run Shaw HospitalHangzhou Neoantigen Therapeutics Co., Ltd.NCT05269381Personalized Neoantigen Peptide-Based Vaccine in Combination With Pembrolizumab for Treatment of Advanced Solid TumorsMayo ClinicNCT05098210Personalized Neo-Antigen Peptide Vaccine for the Treatment of Stage IIIC-IV Melanoma or Hormone Receptor Positive Her2 Negative Metastatic Refractory Breast CancerFred Hutchinson Cancer CenterAmazon.com Services LLCNCT05743595Neoantigen-based Personalized DNA Vaccine With Retifanlimab PD-1 Blockade Therapy in Patients With Newly Diagnosed, Unmethylated GlioblastomaWashington University School of MedicineIncyte CorporationNCT04397003Personalized Neoantigen Vaccine in Combination With Durvalumab (MEDI4736) in Extensive Stage Small Cell Lung CancerWashington University School of MedicineAstraZeneca|Gateway for Cancer ResearchNCT04024878NeoVax With Nivolumab in Patients With Ovarian CancerDana-Farber Cancer InstituteUnited States Department of DefenseNCT03929029Neoantigen Vaccine Plus Locally Administered Ipilimumab and Systemic Nivolumab in Advanced MelanomaDana-Farber Cancer InstituteNational Cancer Institute (NCI)NCT05631886Combination of CAR-DC Vaccine and ICIs in Malignant TumorsChinese PLA General HospitalZhejiang UniversityNCT03897881An Efficacy Study of Adjuvant Treatment With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Participants With High-Risk Melanoma (KEYNOTE-942)ModernaTX, Inc.Merck Sharp & Dohme LLCNCT03631043Personalized Vaccine in Treating Patients With Smoldering Multiple MyelomaM.D. Anderson Cancer CenterNCT05981066A Clinical Study of mRNA Vaccine (ABOR2014/IPM511) in Patients With Advanced Hepatocellular CarcinomaPeking Union Medical College HospitalNCT04930783NeoVax + CDX-301 and Nivolumab in MelanomaDana-Farber Cancer InstituteCelldex TherapeuticsNCT06095934Efficacy and Safety of Neoantigen Peptide Vaccine in the Treatment of Advanced NSCLC Progressed After EGFR-TKI TreatmentThe Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical SchoolNCT05013216Mutant KRAS -Targeted Long Peptide Vaccine for Patients at High Risk of Developing Pancreatic CancerSidney Kimmel Comprehensive Cancer Center at Johns HopkinsStand Up To CancerNCT03606967Testing the Addition of an Individualized Vaccine to Nab-Paclitaxel, Durvalumab and Tremelimumab and Chemotherapy in Patients With Metastatic Triple Negative Breast CancerNational Cancer Institute (NCI)NCT05579275Evaluate the Safety and Tolerability of JCXH-212 Injection in the Treatment of Advanced Malignant Solid TumorsPeking University Cancer Hospital & InstituteNCT05641545IVAC-RCC-001: A Personalized Neoantigen Vaccine as Add-on to Standard of Care Checkpoint Inhibitor in Advanced/Metastatic RCC PatientsSLK Kliniken Heilbronn GmbHNCT05685004Study of Neoantigen-specific Adoptive T Cell Therapy for Newly Diagnosed MGMT Negative Glioblastoma Multiforme (GBM)TVAX BiomedicalNCT05354323NECVAX-NEO1 in Addition to Checkpoint Inhibitor in Patients With Solid TumorsNEC OncoImmunity ASNCT02600949Personalized Peptide Vaccine in Treating Patients With Advanced Pancreatic Cancer or Colorectal CancerM.D. Anderson Cancer CenterNational Cancer Institute (NCI)NCT05886439LK101 Combined With PD-1 or PD-L1 Monoclonal Antibody in the Treatment of Lung CancerCancer Institute and Hospital, Chinese Academy of Medical SciencesNCT05631899Combination of CAR-DC Vaccine and ICIs in Local Advanced/Metastatic Solid TumorsChinese PLA General HospitalZhejiang UniversityNCT04943718Personalized Vaccine for Patients With Recurrent Malignant GliomaXuanwu Hospital, BeijingBeijing Neoantigen Biotechnology CompanyNCT04943848rHSC-DIPGVax Plus Checkpoint Blockade for the Treatment of Newly Diagnosed DIPG and DMGAnn & Robert H Lurie Children's Hospital of ChicagoDana-Farber Cancer Institute|Children's Hospital of Orange County|University of Calgary参考文献:
I.E. Shapiro and M. Bassani-Sternberg,The impact of immunopeptidomics: From basic research to clinical implementation,Semin Immunol. 2023 Mar:66:101727.
L.E. Stopfer, A.D. D’Souza, F.M. White, 1,2,3, MHC: a review of massspectrometry-based immunopeptidomics methods for relative and absolute quantification of pMHCs, Immuno-Oncol. Technol. 11 (2021), 100042, https:// doi.org/10.1016/j.iotech.2021.100042. D. Gfeller et al. Contemplating immunopeptidomes to better predict them,Semin Immunol. 2023 Mar:66:101708.Santambrogio L and Franco A (2022) The yin/yang balance of the MHC-self-immunopeptidome. Front. Immunol. 13:1035363. doi: 10.3389/fimmu.2022.1035363Clement CC, Nanaware PP, Yamazaki T, Negroni MP, Ramesh K, Morozova K, et al. Pleiotropic consequences of metabolic stress for the major histocompatibility complex II molecule antigen processing and presentation machinery. Immunity (2021) 54:721–736 e10. doi: 10.1016/j.immuni.2021.02.019] N. Hilf, et al., Actively personalized vaccination trial for newly diagnosed glioblastoma, Nature 565 (7738) (2019) 240–245.医脉通是专业的在线医生平台,“感知世界医学脉搏,助力中国临床决策”是平台的使命。医脉通旗下拥有「临床指南」「用药参考」「医学文献王」「医知源」「e研通」「e脉播」等系列产品,全面满足医学工作者临床决策、获取新知及提升科研效率等方面的需求。
